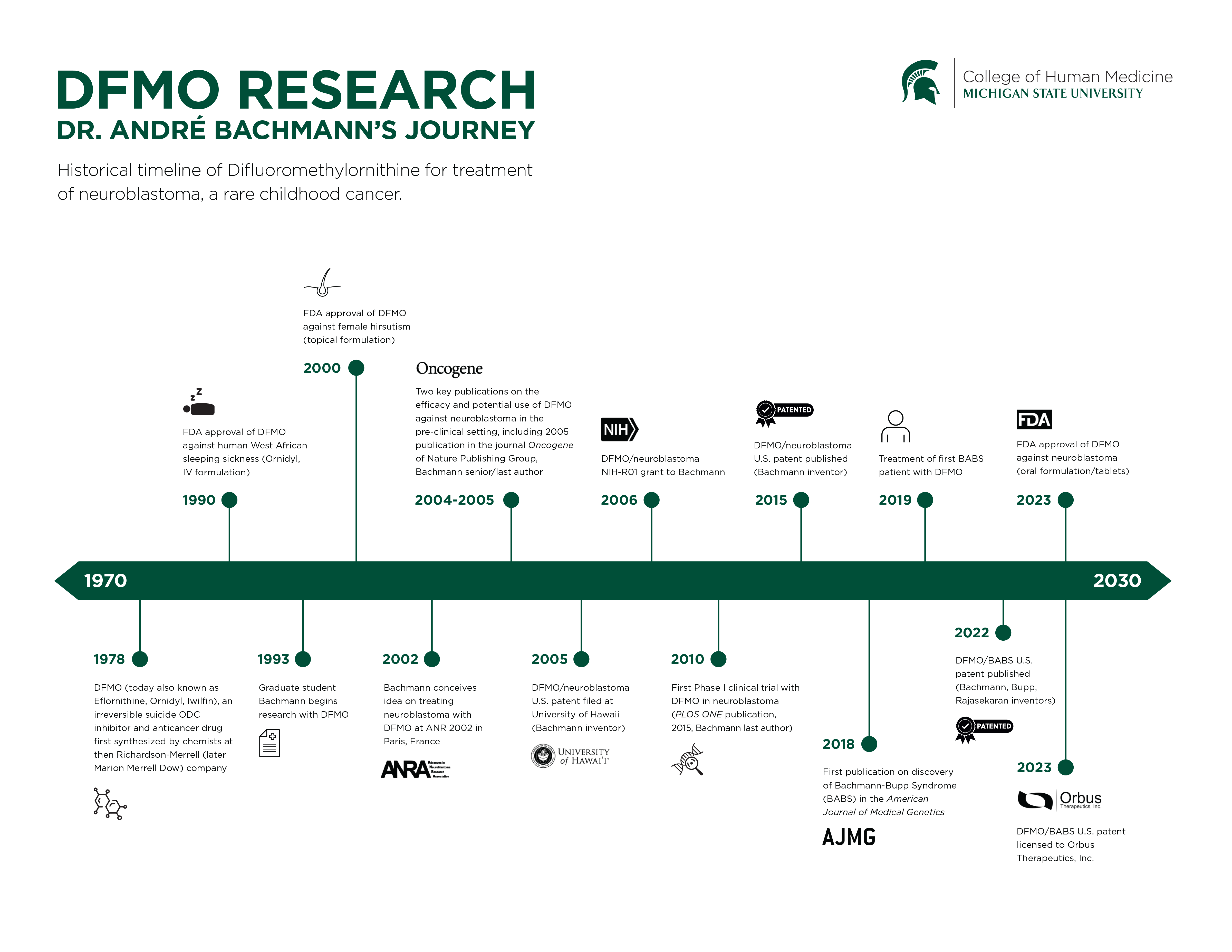
The U.S. Food and Drug Administration has approved a drug to treat neuroblastoma, an often-fatal pediatric cancer, based on pioneering research by College of Human Medicine professor André Bachmann.
The FDA approved a tablet form of a drug called difluoromethylornithine, or DFMO (synonym eflornithine), developed in 1978 and later used to treat West African sleeping sickness. The agency’s approval will allow doctors to use oral DFMO to reduce the rate of relapses in patients who have previously undergone standard therapy for neuroblastoma.
 “This is an extraordinary milestone,” said Bachmann, PhD, a professor and associate chair for research in the College of Human Medicine’s Department of Pediatrics and Human Development, who had been studying DFMO as a potential treatment for neuroblastoma for more than 20 years.
“This is an extraordinary milestone,” said Bachmann, PhD, a professor and associate chair for research in the College of Human Medicine’s Department of Pediatrics and Human Development, who had been studying DFMO as a potential treatment for neuroblastoma for more than 20 years.
Each year, about 700 children in the United States, most of them age 5 or younger, are diagnosed with the extremely aggressive tumor, which forms on the nerve cells in several areas of the body. It accounts for about 12% of all childhood cancer deaths.
In the past, few patients survived neuroblastoma if the cancer returned after going into remission.
Bachmann has studied DFMO and its target protein ornithine decarboxylase (ODC) since 1992. His interest in DFMO as a potential treatment for neuroblastoma began in 2002 when, at a conference in Paris, he sat with grieving parents whose children had died or were still fighting neuroblastoma.
“That’s when I realized how frustrated all these parents are, because it can take 15-20 years and large amounts of money to develop a new drug,” he said, “and I thought, why not try to repurpose a drug that is already used in the clinic for another disease?”
DFMO, he later realized, could be a potential treatment, because it targets ODC, which when elevated in the body promotes the growth of neuroblastoma tumor cells. That initial discovery led to a series of preclinical research by Bachmann’s group, which was funded by a National Cancer Institute R01 grant in 2006 and by several other awards, including one from the St. Baldrick’s Cancer Foundation. His early preclinical research findings, together with additional contributions by his collaborators and colleagues at other institutions, were convincing and led to the first neuroblastoma clinical trial with DFMO in 2010. This phase I study, as well as subsequent phase II relapse prevention studies with DFMO, were spearheaded by the Corewell Health Helen DeVos Children’s Hospital in Grand Rapids. Today, several independent multi-center phase II clinical trials are ongoing at scores of pediatric hospitals all over the country.

The fact that the FDA previously had approved DFMO as a treatment for another disease and that it had few side effects could significantly shorten the approval process, Bachmann reasoned, and this was confirmed in the neuroblastoma phase I clinical trial with DFMO, a study that was published in 2015.
Data from the phase II relapse clinical trials showed that DFMO reduced the risk of relapse by 50%, an organization called Beat Childhood Cancer reported. Based on the results of those trials, an FDA panel of experts called the Oncologic Drugs Advisory Committee voted 14-6 on Oct. 4, 2023, to recommend that the agency grant final approval.
The FDA on Dec. 13 granted final approval for DFMO to reduce the risk of relapse in adult and pediatric patients with high-risk neuroblastoma.
Bachmann was modest about his contributions.
“I want to be clear – I don’t want to take credit for a lot of work other people did, especially towards the later stages in the clinic,” he said. “What I did was preclinical work with participation in the early phase I trial. This became much bigger than my lab. Hundreds of talented people that deserve credit have contributed to this, but I am probably most proud to say that I was the guy who lit the match at a time when the neuroblastoma clinical world did not consider DFMO for treatment and successfully brought this potential therapy to the attention of pediatric oncologists.
“I am humbled to have played some role in this.”
This story was originally published on College of Human Medicine.
About the MSU Innovation Center:
The MSU Innovation Center is dedicated to fostering innovation, research commercialization, and entrepreneurial activities from the research and discovery happening across our campus every day. We act as the primary interface for researchers aiming to see their research applied to solving real-world problems and making the world a better place to live. We aim to empower faculty, researchers, and students within our community of scholars by providing them with the knowledge, skills, and opportunities to bring their discoveries to the forefront. Through strategic collaborations with the private sector, we aim to amplify the impact of faculty research and drive economic growth while positively impacting society. We foster mutually beneficial, long-term relationships with the private sector through corporate-sponsored research collaborations, technology licensing discussions, and support for faculty entrepreneurs to support the establishment of startup companies.
Is your company looking to fund further research by MSU’s College of Human Medicine? Click Here.