MSU Professor Lawrence Drzal leads the charge on a nanomaterial called graphene that could be in the next generation of EV batteries
Most people are familiar with graphite, the dark silver-colored crystalline form of carbon commonly used in pencil lead. In fact, graphite derives its name from its usage (graphein is “to write” in Greek).
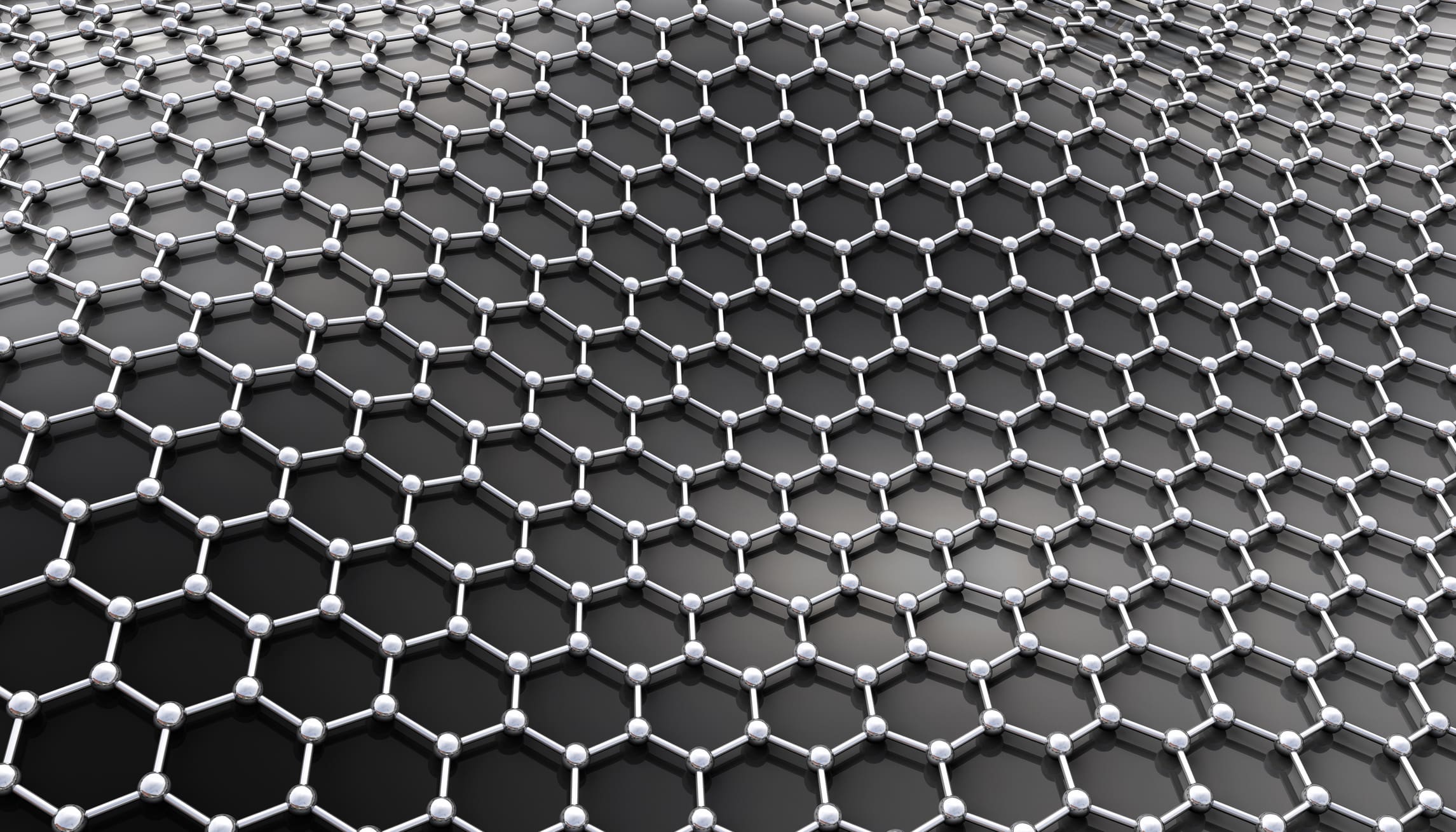
What’s so special about graphite? When you zoom in on graphite with a high-powered electron microscope, you can see a surface layer that is made up of carbon atoms arranged in a regular hexagonal pattern. This layer is one sheet that is stacked on many other identical sheets and forms graphite. The hexagonal carbon atoms in an individual sheet of graphite are held together with a very strong bond, but the graphite sheets that are stacked on each other are only held together with a much weaker bond. Because of this weak bonding between graphite sheets, they can easily slide over each other and be separated.
Beginning as early as the 1800s, scientists started experimenting with graphite particles and exploiting the element’s unique properties. In 1987, the word “graphene” was first given as the name for single sheets of graphite—just one atom thick—and researchers continued to manipulate it and find novel uses for the unusual material over the ensuing decades.
In the early 2000s, scientists from the United Kingdom and Russia extracted thin layers of graphite from a crystal using Scotch tape. They were able to transfer those layers to another surface. They wrote a paper on graphene’s remarkable flexibility, strength, and electrical conductivity and earned a Nobel Prize in 2010. Research into modern-day uses for graphene began to increase significantly.
MSU Professor Enters Graphene Frontier

Enter Lawrence Drzal, Distinguished Professor of Chemical Engineering and Materials Science at Michigan State University (MSU), who by 2003 had been working extensively with carbon fibers and polymers to produce composites (materials formed by putting two different materials together in a way that retains each material’s identity). He immediately saw the opportunity for graphene as a nanomaterial that could add new properties if added to polymers and composites.
“I talked with my graduate students and said, ‘Hey, let’s see if we can develop an inexpensive process to make graphene from graphite, use it as a reinforcement and improve the mechanical properties of plastics, and use graphene to add additional properties to polymers and composites,” says Drzal. “That was the start of our journey into investigating the wonders of graphene.”
“The most interesting part was the graphene itself is electrically conductive, it’s thermally conductive, it’s a large platelet, and it is very thin — a two-dimensional platelet. If I put it on a surface or in a material, other molecules can’t go through it; they have to go around it,” Drzal says. “Because of its 2D nature, only a very small concentration of graphene particles are necessary to touch each other and form a thermally or electrically conductive network.” All those things made it very, very attractive. On top of all that, it was a material that has very low density, so it’s not something that you add, and it makes the whole structure very heavy.”
Drzal and his group found a highly attractive application for graphene in the area of carbon fiber polymer composites, which are already used in many applications: rockets, aircraft, vehicles, construction materials, etc. Polymer composites are made with carbon fibers (smaller in diameter than a strand of human hair) and surround them with a polymer (plastic) to create a composite.
“Think of the graphene-like a stack of plates in a cabinet, for example,” he says. “They’re all the same; they fit nicely on top of one another. And if you pick up that stack, it’s got the properties of the whole stack, but it would be very difficult to make something from this stack of plates. Now what I want to do is to be able to stick the plates together. Putting a polymer around all of the plates glues the plates together, and then I can make something with that stack of plates.”
“But now, with graphene, I am doing it at a level that’s nanoscopic in size. Graphene sheets are only one atom thick, but the beauty of them is that they can be made very large. They can be made a thousand times as wide as their thickness.” Creating these materials and developing methods to separate the individual graphene sheets, modify their surfaces and develop ways to disperse and organize them in polymers and composites was the basis of Drzal’s work that continues today.
Potential Uses in Batteries, Other Applications
What excites Drzal and other scientists and engineers about graphene is the unique carbon arrangement of graphene, which can be organized for a multitude of applications: for example, the potential for using graphene in electric vehicles. Ford Motor Company is one of many corporations using graphene for various commercial applications.
“We’d like to be able to make sure that those battery boxes that are going to be powering our electric vehicles (EVs) don’t emit stray electromagnetic radiation, so we need to put them in a conductive box. The question is, how do we make that? Well, if we make it out of steel or some other material, it will be conductive, but it will be very heavy,” says Drzal.
“The batteries required for a reasonable EV range are quite heavy. I don’t want to add more weight with a metal battery box, but now I can use a lightweight polymer composite and add just a couple of percent of these graphene platelets to the composite. Remember: they’re very large, like a plate, but very thin. If I only add about 1 percent of them by weight into the plastic, they’ll actually touch each other. For electrical conductivity, all I need is a path for the electrons to go through the polymer by moving from one platelet to another. I can make the polymer very conductive by adding only a small amount of graphene material. The same approach works for thermal conductivity,” he says.
The strength of graphene plays an important role, too. “We want the battery box to be strong and tough in case of a collision so that they can absorb energy without fracturing,” says Drzal.
MSU Innovation Center Supports Graphene Intellectual Property Development
In 2006, seeing some of the tremendous potential of graphene, Drzal, along with some of his former graduate students, co-founded XG Sciences, Inc. He served as the chief scientist in this East Lansing-based graphene nanoplatelet startup company. With the help of MSU Technologies, the technology transfer office at MSU, XG Sciences licensed several graphene technologies from MSU. XG Sciences annually manufactured and sold tens of thousands of pounds of xGnP® brand graphene nanoplatelets. In 2022, most of the assets of XG Sciences were acquired by NanoXplore, a Canadian company active in the graphene market.
Drzal says that the MSU Innovation Center and the environment at MSU for supporting research and intellectual property is ideal for fostering connections between scientists and business partners—an environment from which he’s benefited.
“This was not something that I expected to do in my career, but I will say that the environment that Michigan State has, especially now with the MSU Innovation Center, is very good for identifying potential developments that can be turned into intellectual property, be patented to jump off into starting businesses or to enhance the ability of existing businesses to get a wider product portfolio,” he says. “I’m very pleased with MSUT in terms of what they do and how they do it, and I think it’s a real asset that MSU has in being able to manage its intellectual property this way.”
During his career, Drzal has published over 400 peer-reviewed research papers and has been awarded 41 patents. He has been identified by the Institute for Scientific Information as one of the most cited materials researchers and was elected as a Fellow of the National Academy of Inventors. He serves on the editorial board of five journals and numerous government committees and has been elected a Fellow of five professional societies.
MSU continues to own an extensive portfolio of the graphene technologies of Professor Drzal and is actively pursuing promotion and licensing opportunities. Click HERE to learn more about MSU’s graphene technology portfolio that are available for licensing, or contact Jon Debling at deblingj@msu.edu
About the MSU Innovation Center:
The MSU Innovation Center is dedicated to fostering innovation, research commercialization, and entrepreneurial activities from the research and discovery happening across our campus every day. We act as the primary interface for researchers aiming to see their research applied to solving real-world problems and making the world a better place to live. We aim to empower faculty, researchers, and students within our community of scholars by providing them with the knowledge, skills, and opportunities to bring their discoveries to the forefront. Through strategic collaborations with the private sector, we aim to amplify the impact of faculty research and drive economic growth while positively impacting society. We foster mutually beneficial, long-term relationships with the private sector through corporate-sponsored research collaborations, technology licensing discussions, and support for faculty entrepreneurs to support the establishment of startup companies.
Is your company interested in sponsoring further graphene research on campus? Click Here.